The cooling of a body
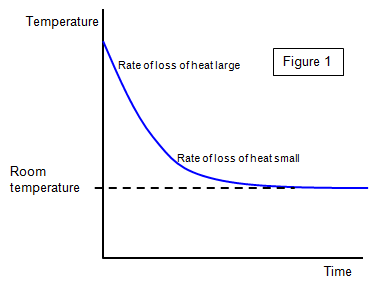
In this section we will think about what happens in any experiment where heat is
added to a body. The body will gain energy but it will also lose energy to the surroundings. In
fact the bigger the temperature difference between an object and its surroundings the greater
will be the rate of loss of heat energy. This becomes zero when the object reaches the same
temperature as its surroundings. You can see an example of this is Figure 1.
The
law governing the rate of loss of heat from a body to its surroundings was first proposed by
Newton in 1701 and is therefore known as Newton's law of cooling. He proposed that:
The rate of loss of heat of a body by cooling in a steady stream of air is proportional to the excess temperature (θ - θs) of the body above its surroundings.
This can
be expressed mathematically as:
- dH/dt = k(q - qs)
and if C is the thermal capacity of the
body as:
- Cdθ/dt = k(θ - θs)
This has been found to hold very well for forced convection, where the air velocity is >4
ms
-1 but not too well in still air, that is, for natural convection.
For natural
convection the law has been shown experimentally to be a 5/4 power law.
That is:
- dH/dt = k(θ - θs)5/4
The following
set of results may be used to investigate the law of natural convection.
| Excess temperature (oC) |
20 |
30 |
40 |
50 |
60 |
70 |
| Rate of loss of heat (Js-1) |
0.212 |
0.350 |
0.501 |
0.660 |
0.830 |
1.01 |
| lg(excess temperature) |
1.301 |
1.477 |
1.602 |
1.699 |
1.778 |
1.845 |
| lg(rate of loss of heat) |
-0.674 |
-0.456 |
-0.300 |
-0.180 |
-0.081 |
0.004 |
If a graph is
plotted of lg(excess temperature) against lg(rate of heat loss) the validity of the law can be
checked.
Heat losses can be useful, in fact essential in some cases. Engines are
cooled with water or air and integrated circuits that work at relatively high power are set in a
piece of blackened aluminium.
A VERSION IN WORD IS AVAILABLE ON THE SCHOOLPHYSICS CD