Specific latent heat of vaporisation of a liquid
AIM
The aim of this experiment is to measure the specific latent heat of vaporisation of a liquid.
YOU WILL NEED
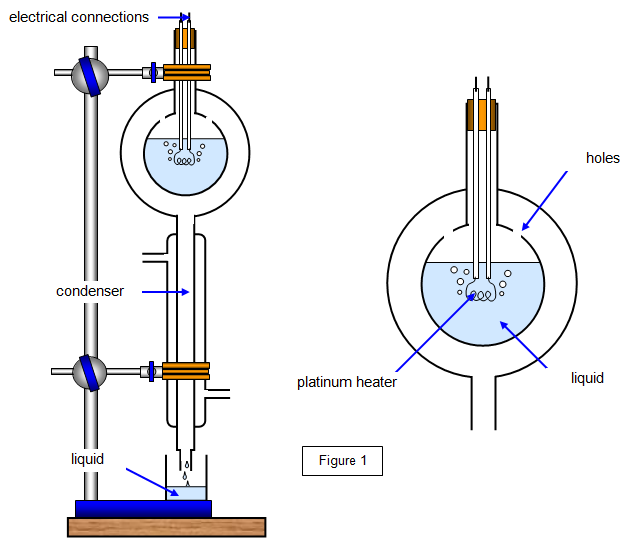
The special latent heat apparatus shown in the diagram, a low voltage power supply, a volatile liquid, two small (100 ml) beakers, access to a balance, a retort stand boss and clamp, a joule meter or an ammeter, voltmeter and a stopwatch
WHAT TO DO
Set up the apparatus as shown in Figure 1. Bring the liquid to the boil. When the liquid is dripping steadily out of the condenser collect the mass m) that condenses in a time t.
Use either a joulemeter or a voltmeter and ammeter to calculate the electrical energy input (VIt) in that time.
ANALYSIS AND CONCLUSIONS
Hence calculate the specific latent heat of vaporisation (L) from the equation
VIt = mL.
Note: These instructions are only intended as an outline of experimental procedure. You should consult your teacher for a more detailed version before carrying out the experiment.
 DO NOT ALLOW THE HEATER TO HEAT UP UNLESS IT IS WELL COVERED BY THE LIQUID. DO NOT ALLOW THE APPARATUS TO BOIL DRY. BE AWARE OF THE FIRE HAZARD OF A VOLATILE LIQUID.
DO NOT ALLOW THE HEATER TO HEAT UP UNLESS IT IS WELL COVERED BY THE LIQUID. DO NOT ALLOW THE APPARATUS TO BOIL DRY. BE AWARE OF THE FIRE HAZARD OF A VOLATILE LIQUID.