

An aluminium block, heat resistant mat on which to place the block, suitable lagger to cover the block, an electrical immersion heater, a voltmeter, an ammeter, connecting leads, a low voltage power supply, a thermometer (0 – 50oC), a stop watch.
Measure the mass of the aluminium block (MA).
Record the initial temperature of the block (θ1).
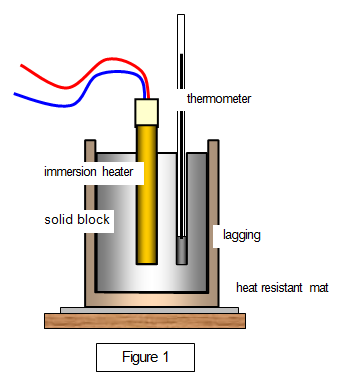
Set up the apparatus as shown in Figure 1.
Switch on the heater allow it to heat up so that it is slightly warm to the touch and then put it in the hole in the block.
Leave the heater switched on and in the aluminium block for a measured time (t) (usuaully between five and ten minutes for a 1kg block and a 60W heater) recording the temperature of the block at fifteen second intervals.
Switch off the heater and record the final temperature of the aluminium (θ2).
Record the voltage (V) and current (I), this may need to be adjusted throughout the experiment so that the power input remains constant.
Record any sources of error which you consider will affect your result and suggest how they might be reduced.
Plot a graph of the temperature of the block against time and use it to obtain a more accurate value for the specific heat capacity of aluminium.
Repeat the experiment with a block of different metal.

| SAFETY CONSIDERATIONS: The block of aluminium will get hot during the experiment |