
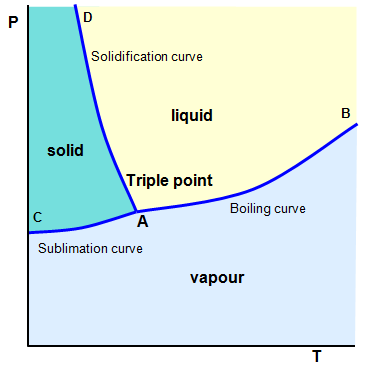
If the graph is for water then
the three lines shown represent:
(a) the boiling curve (AB) is the locus of points where
water and its vapour can exist in equilibrium;
(b) the sublimation curve (AC) is the locus
of points where ice and its vapour can exist together in equilibrium;
(c) the solidification
curve (AD) is the locus of points where water and ice can exist together.
The triple
point is at A where these three lines intersect and where water, water vapour and ice can
exist in equilibrium. The temperature at which this occurs is defined as 273.16 K on the
thermodynamic temperature scale. (The triple point is not exactly at 0 oC because under the
pressure of its own vapour ice melts at about 0.0075 oC.)