
(a)
Specific heat capacity at constant volume (cp)
The specific heat capacity of a gas at
constant volume (cV), can be measured using the differential steam calorimeter invented by
Joly in 1886 (Figure 1).
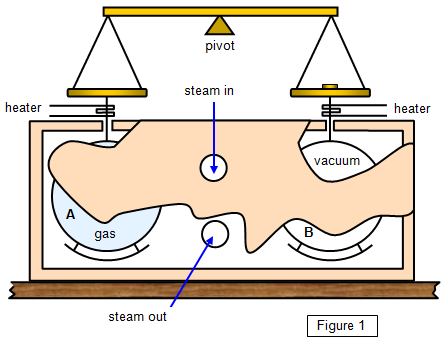
Two hollow copper spheres identical in
size and mass are suspended by wire from the two pans of a beam balance. One of the
spheres contains a mass of gas M at high pressure (Joly used 22 atmospheres) and the
other is evacuated. The spheres are surrounded by a box through which steam is
passed.
Steam will condense on the cool spheres, more steam condensing on the
one with the greater thermal capacity, that is, the one filled with gas. After some time a mass
m has to be placed on pan B to counterbalance the extra mass of steam that has condensed
on sphere A.
If cV is the specific heat capacity of the gas at constant volume and
θo and q1 the
initial and final temperatures of the sphere, then:
cV = mL/[M(θ1 -θo)]
where L is the specific heat of vaporisation of
water.
The two wires are heated to vaporise any liquid condensing on them, and
baffles are provided to prevent water dripping off the roof of the box on to the spheres. A
control experiment is performed to check the amount of steam condensing on the spheres
when both are empty.
(b) Specific heat capacity at
constant pressure (cP)
The specific heat capacity at constant pressure
cP can be measured using the continuous-flow calorimeter devised in 1862 by
Regnault. The modern method is a modification of the constant-flow method first used by
Swann in 1909.
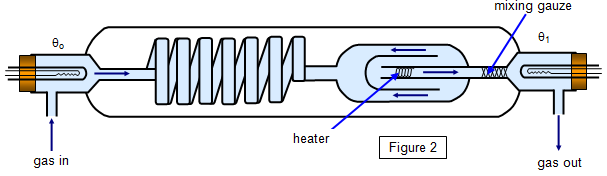
The
apparatus is shown in Figure 2. Gas is passed round a series of tubes in a water bath to
ensure that it has reached a constant temperature and is then fed into the apparatus at A.
Here its temperature is measured using a platinum resistance thermometer (θo). It passes over a heater where its temperature is
raised, then through a mixing gauze and finally out at B where its temperature is measured
again (θ1).
When steady state has been
reached the temperatures are recorded, and hence cP can be calculated from
VIt =
McP(θ1 – θo) + H
where VIt is the energy supplied to the
heater in a time t, M is the mass of gas passing through in that time and H is the heat lost.
As usual with continuous-flow experiments, by doing two measurements with the same
temperature difference but with a different rate of flow H can be eliminated.