
This famous experiment performed in 1852 was a follow-up
to those of Gay-Lussac (1807) and Joule (1845) and demonstrated that there were indeed
attractive forces acting between gas molecules. In theory, if attractive forces do exist then
when a gas expands its temperature should drop. The potential energy of the gas molecules
has been increased and therefore in an isolated system its kinetic energy, and thus its
temperature, should fall.
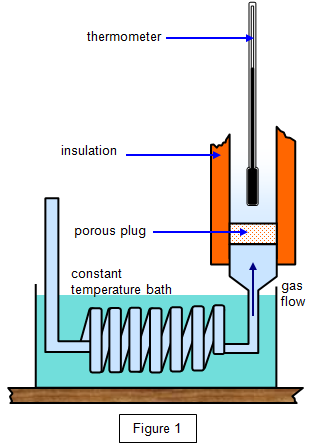
The apparatus used is shown in Figure 1. The
experiment is often known as the porous plug experiment because gas at high pressure was
allowed to expand through a cotton wool plug. The plug prevented eddies forming and the
gas did not gain any kinetic energy in bulk. The initial temperature of the gas was maintained
by the constant-temperature bath.
All gases showed a
temperature change when passing through the plug but for some it was a cooling and for
others a heating. The change in temperature was proportional to the pressure difference
between the two sides of the plug: this can be understood if it is realised that work is done
on the gas in forcing it through the plug and by the gas when it expands on emerging. For every gas there is
an inversion temperature; if the initial temperature of the
gas is above this then heating occurs and if it is below this cooling.
For helium
this inversion temperature is 30 K, for hydrogen 190 K and for most other gases it is well
above room temperature.
The table below gives the temperature changes per
atmosphere observed in the experiment.
| Gas | Temperature change (oC atm-1) |
| Air | -0.208 |
| Carbon dioxide | -1.005 |
| Hydrogen | -0.039 |
| Nitrogen | -0.249 |
| Oxygen | -0.253 |