
The amount of water vapour in the atmosphere is known as its humidity. The ease with which our bodies lose water vapour depends on the humidity of the air, that is, how much water vapour is already present in it. Water vapour will condense from the air when it is saturated, and since saturation varies with temperature cooling down a sample of air will often result in condensation occurring. The formation of clouds and rain is governed by these effects. If the humidity is high it means that the atmosphere contains a lot of water vapour. It is therefore difficult for water vapour to evaporate from our bodies and so we sweat more freely.
The formation of a cloud in the cloud
chamber is caused by cooling the air containing meths vapour (by expansion or by the use
of 'dry ice') to a point where it becomes saturated and liquid is forced to
condense.
The humidity of the atmosphere is measured by an instrument known as
a hygrometer, the most common types of this being the wet and dry bulb hygrometer and the
hair hygrometer.
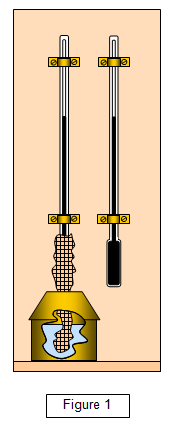
A simplified diagram of a wet and dry bulb hygrometer is shown in
figure 1.
The wet and dry bulb hygrometer consists of two thermometers, on of
which has its bulb wrapped in muslin. The muslin is kept wet by its lower part being
immersed in a small can of water. Evaporation occurs from the muslin and so the
temperature recorded by the 'wet bulb' is lower than that recorded by the dry bulb. Since the
rate of evaporation will depend on the humidity of the atmosphere the difference in
temperature between the two thermometers can be used to find the relative
humidity.
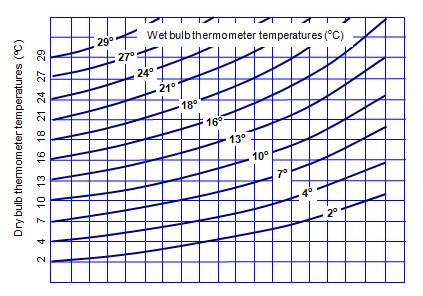
Knowing the wet and dry bulb temperatures the relative humidity can be
found.
If you wear glasses you will know that if you put them on when lying in a hot
bath the glasses steam up. The glasses are cold compared with the air around them and so
water vapour condenses on them. They will only clear if you keep wiping them or warm them
up.
Dew forms on the grass during a cold night following a relatively warm day.
The cold air at night cannot hold as much moisture as it could during the day and so some
must condense out as dew. If the night is realty cold this dew freezes and you get
frost.
At the dew point there is just enough water vapour in the air to saturate
it.
The dew point can be found using Regnault's dew point hygrometer.