
The specific latent heat of vaporization of a liquid may be measured by a modification of the method of Ramsey and Marshall (1896). The apparatus is shown in Figure 1.
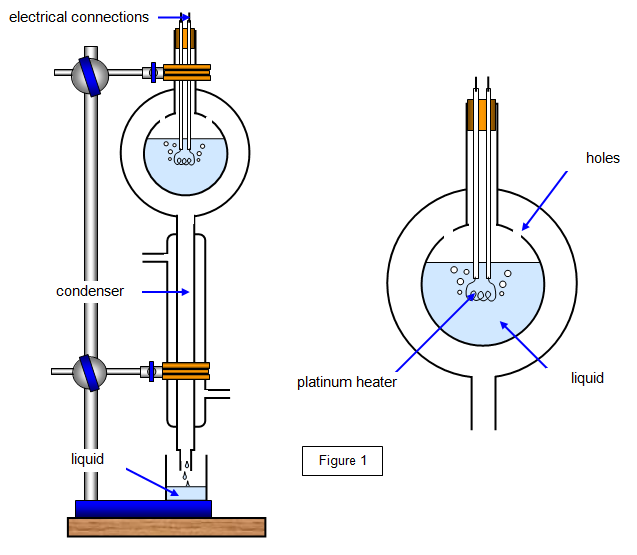
The double-walled glass vessel is fitted to a condenser and mounted
vertically. The inner section contains the liquid in which is a heater made of platinum wire. When
the liquid boils the vapour passes through small holes into the outer vessel and then down into
the condenser. Here it condenses, runs down and is collected in the beaker. It is essential that
evaporation is rapid, for then the vapour in the outer vessel acts as a heat shield and eliminates
heat losses from the inner vessel.
When a steady state has been reached - that is,
when liquid drips into the beaker at a constant rate - a clean beaker is placed under the
condenser and the mass of liquid m condensing, and hence being evaporated, in a measured
time t can be found.
The specific latent heat of vaporisation L of the liquid can then be found
from the equation
where VI is
the power supplied to the coil. If a joule-meter is available the energy input E may be measured
directly; then
Energy input (E) = mL
The large specific latent heat of vaporization of water
explains why it is much more painful to be scalded by steam at 100 oC than by an equal
mass of liquid water at 100 oC. The steam first condenses before it cools to your body
temperature and in doing so releases roughly ten times as much heat energy as it does in the
cooling phase.
The simplest method for measuring this quantity is the method of mixtures. Ice is
dropped into water a few degrees above room temperature, and the resulting fall in temperature
is recorded after all the ice has melted. Since the water falls from a few degrees above the
temperature of the surroundings to a few degrees below the heat losses may be ignored - the
mixture is assumed to gain as much heat as it losses and a cooling correction need not be
applied.
(See also:
Latent heat of fusion experiment