
For solids and liquids we define
the specific heat capacity as the quantity of energy that will raise the temperature of unit
mass of the body by 1 K. For gases, however, it is necessary to specify the conditions under
which the change of temperature takes place, since a change of temperature will also
produce large changes in pressure and volume.
For solids and liquids we can
neglect this pressure change and the specific heat capacity that we measure for them is
essentially one where the pressure on the body is unaltered. We call this the specific heat
capacity at constant pressure (CP).
The specific heat capacity of a gas will
depend on the conditions under which it is measured and since these could vary
considerably we will restrict ourselves to the following, called the principal specific heat capacities of a gas:
(a) The
specific heat capacity at constant volume (cv) is defined as the quantity of heat
required to raise the temperature of 1 kg of the gas by 1 K if the volume of the gas remains
constant.
(b) The specific heat capacity at constant pressure (cp) is
defined as the quantity of heat required to raise the temperature of 1 kg of the gas by 1 K if
the pressure of the gas remains constant.
The specific heat capacity at constant
pressure (cp) is always greater than that at constant volume (cv),
since if the volume of the gas increases work must be done by the gas to push back the
surroundings.
The molar heat capacity at
constant volume (CV) is the quantity of heat required to raise the temperature
of 1 mole of the gas by 1 K if the volume of the gas remains constant. The molar heat
capacity at constant pressure (CP) is the quantity of heat required to raise the
temperature of 1 mole of the gas by 1 K if the pressure of the gas remains
constant.
The table below gives the principal specific heat capacities for some well-known gases.
| Gas | Specific heat capacity atconstant pressure (J kg-1K-1) | Specific heat capacity atconstant volume (J kg-1K-1) |
| Air | 993 | 714 |
| Argon | 524 | 314 |
| Carbon dioxide | 834 | 640 |
| Carbon monoxide | 1050 | 748 |
| Helium | 5240 | 3157 |
| Hydrogen | 14300 | 10142 |
| Nitrogen | 1040 | 741 |
| Oxygen | 913 | 652 |
| Water vapour | 2020 | - |
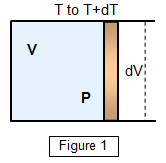
If we now return to the
initial conditions and heat the gas again but this time allow it to expand, keeping the
pressure constant, the energy input for a temperature rise of dT is
CVdT.
This not only has to raise the temperature of the gas but also
must do external work in expanding it by dV. Therefore
CPdT =
CVdT + dW
CPdT = CVdT +
PdV
We assume that the gas obeys the ideal gas equation for one mole PV = RT,
and therefore PdV = RdT. Substituting for dV we have: