
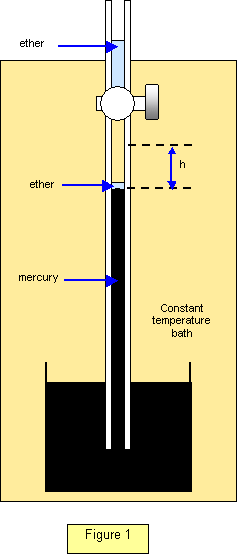
The saturated vapour pressure (s.v.p.) of a liquid - ether, for
instance - may be measured using the apparatus shown in Figure 1.
Ether is
introduced into the space above the mercury in a barometer tube, using a special pipette,
and the resulting depression of the mercury column is measured. The drop in level (h) is the
saturated vapour pressure of ether at that temperature. For an accurate determination we
should allow for the effects of the weight of excess ether and for its surface tension, although
these are small and are usually neglected. The barometer tube should also be kept in a
water bath at a constant temperature.
Generally the more volatile the liquid the
greater its s.v.p at a given temperature.
The s.v.p. of ether at 20
oC is 44 cm of mercury and this falls to 36 cm of mercury at 18
oC.
The corresponding values for water at these temperatures are 1.8
cm and 1.5 cm, while for mercury the s.v.p. at 20 oC is only 1.2x10-3
mm.
It can be shown by consideration of surface tension effects that droplets of liquid always tend to evaporate, due to the excess pressure at their surfaces, and that small drops evaporate faster than large ones. If this is the case, how do droplets condense at all? In fact they find it very difficult unless there is a nucleus, such as a dust particle or a charged ion, on which the drop can begin to grow. This means that the pressure of a vapour can exceed its saturated vapour pressure at a given temperature before condensation takes place. Such a vapour is said to be in a supersaturated state. Condensation will begin rapidly if a suitable nucleus is introduced into the vapour; this is of great importance in the cloud chamber.