
The measurement of the velocity
of sound in a gas is the most convenient way of finding γ, but a direct method is described
below.
This method was devised by Clement and Desormes in 1819. It is based
upon the adiabatic expansion of a gas in a large flask. A laboratory version of their
apparatus is shown in Figure 1.

A large glass flask of at least 10 litres
(0.01 m3) capacity contains air at atmospheric pressure. The flask is fitted with a
large bung containing a bicycle tyre valve, a large stopcock and a tube connecting the flask
to a manometer filled with a light oil. The flask is usually surrounded by insulation although
this is really unnecessary since the air is a bad conductor of heat and the resulting
expansion is rapid. A little concentrated sulphuric acid may be placed in the flask to dry the
air.
The pressure in the flask is raised using the bicycle pump until the manometer
reads a pressure 10-15 cm of oil above that of the surroundings (C-A). The valve is then
closed and the flask allowed to stand until the manometer levels are steady and the air in the
flask has regained its original temperature. The manometer head h1 is recorded. The
stopcock is then opened for a second or two and then closed rapidly. The air in the flask
expands adiabatically (A-B), the temperature drops and the pressure drops to atmospheric
causing the manometer level to fall. The flask is then allowed to stand until the levels
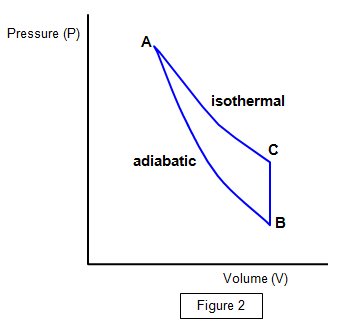
become steady. The new pressure head h2 is then recorded. A PV diagram of the experiment is shown in Figure 2.
It can be shown that if the pressure heads are small
compared with atmospheric pressure then the value of the ratio of the principal specific heat
capacities of the gas γ is given by the equation:
One serious disadvantage of the method is that if the stopcock is large enough to allow a rapid expansion the air inside the flask oscillates, and it is impossible to tell when to close the stopcock since this must be done after one expansion phase only.