Avogadro's law
This important law was proposed by Avogadro in 1811
and then independently by Ampere in 1814. It states that:
Equal volumes of all gases at the same temperature and pressure contain equal numbers of molecules.
This can be verified using the ideal gas
equation. Consider two gases 1 and 2. The gas equation for each gas is:
Gas 1:
P
1 V
1 = n
1RT
1 Gas 2: P
2V
2 =
n
2RT
2Now if P
1 = P
2, V
1 = V
2 and
T
1 = T
2 we have that n
1 = n
2 and so Avogadro's law is
proved.
The volume of one mole of any gas at STP is 22.4 litres. A mole of any substance contains 6.02x1023 molecules Ė a number known as Avogadroís number or Avogadroís constant (NA).
Avogadro's
constant is the number of particles in a mole of the substance. This number is always the
same.
So in:
1 mole of hydrogen (2 g) there are 6.02x10
23 molecules (hydrogen
exists as H
2)
1 mole of oxygen (32 g) there are 6.02x10
23 molecules (oxygen exists as
O
2)
1 mole of copper (63 g) there are 6.02x10
23 atoms
1 mole of uranium 235 (235
g) there are 6.02x10
23 atoms
For example if we have 2 kg of uranium in a fuel rod
we have 2000/235 = 8.51 moles and this contains 8.51x6.02x10
23 = 5.12x10
23 atoms and so
5.12x10
23 uranium nuclei.
Boltzmann's constant (k)
Avogadro's constant is related to another important constant in thermal physics. This is the Boltzmann constant (k) which has a value of 1.38x10
-23 JK
-1.
The Boltzmann constant = R/N
A where R is the universal gas constant (R = 8.31 JK
-1mol
-1).
Dalton's law of partial pressures
If two or more
gases are mixed in a container then the final pressure can be found from Dalton's law,
which states that:
The pressure in a container is the sum of the partial pressures of the gases that occupy the container.
The
partial pressure of a gas is that pressure that the gas would exert if it alone occupied the
container.
For example, consider a container with a volume V and a temperature T. Let
the container be occupied by two different gases, there being n1 moles of gas 1 and
n2 moles of gas 2. Let the partial pressures of the gases be P1 and
P2.
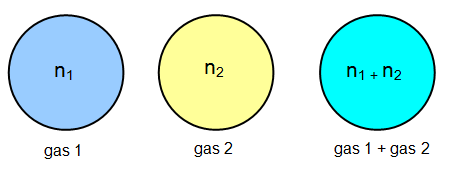
Now
P
1V = n
1RT and P
2V = n
2RT.
But the total pressure in
the container is P, where PV = nRT for n = n
1 + n
2, the total number of
moles of whatever type occupying the container.
Therefore:
P1 =
n1RT/V P2 = n2RT/ V and P= nRT/V and since n =
n1 + n2 we have P1V/RT + P2V/RT = PV/RTor
P = P1 + P2
which is Dalton's
law.
A useful application of this law is in cases where volumes of the same gas
occupy separate but connected containers at differing temperatures.
Example problem

A volume of gas V at a temperature T1 and a pressure p is enclosed in a sphere. It is connected to another sphere of volume V/2 by a tube and stopcock. The second sphere is initially evacuated and the stopcock is closed.
If the stopcock is opened the temperature of the gas in the second sphere becomes T2. The first sphere is maintained at a temperature T1.
Show that the final pressure pí within the apparatus is:
pí = 2pT2/[2T2+T,]
Let there be a molecules of gas, and
let there be n1 molecules in the larger
sphere and n2 molecules in the smaller
sphere after the stopcock is opened.
Now n= n1 + n2, but pV= nRT. So
PV/RT1 = píV/RT1 + píV/2RT2
Therefore: p/T1 = pí(1/T1 + 1/2T2) pí = 2pT2/2T2+T1
A VERSION IN WORD IS AVAILABLE ON THE SCHOOLPHYSICS USB