
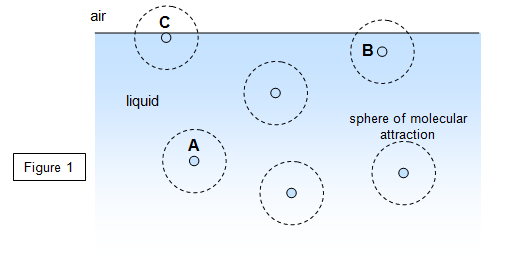
You can easily understand why the surface of a liquid behaves like a skin by looking at Figure 1. A molecule such as A within the body of the liquid will experience a force of attraction due to all other molecules within a small distance of A, this is called the sphere of molecular activity. Molecule A must be in equilibrium and so the resultant force on A is therefore zero. However, a molecule such as B very close to the surface will experience a net inward force; so too will a molecule such as C that is actually in the surface.
The molecules cannot move downwards because there are molecules below them but they do resist being separated from each other, thus giving the skin effect. To call it an elastic skin is misleading, since the surface tension does not vary with the size of the surface as it would in the case of an elastic sheet.
We can use the idea of molecular attraction to explain the shape of a liquid surface near a solid wall such as water in a glass beaker. Figure 2 shows a section of a liquid surface close to the walls of a container.
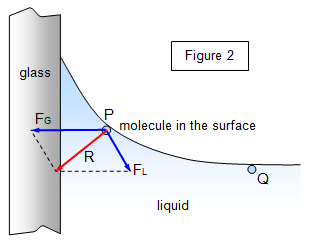
A
liquid molecule at P will experience a force downwards due to gravitational attraction, a force
FL towards the centre of the liquid due to the attractive force of the other liquid
molecules and a horizontal force FG due to the attraction of the molecules of the
material of the walls.
The resultant force R shows that in this case the molecules
will he drawn towards the walls, 'piling up' there to give a concave liquid surface. In the case
of mercury the intermolecular attraction between liquid molecules is greater, and a convex
surface results.