
The properties of a liquid surface give very good evidence for the existence of molecules and tell us quite a lot about the behaviour of these
molecules.
The free surface of a liquid can show many interesting properties due to
a phenomenon known as surface tension. Surface tension explains why liquid drops are
spherical (in the absence of a gravitational field), why water rises up a capillary tube, why
the insects called pond-skaters can walk on water, why your waterproof tent will not let in
rain, why small pieces of camphor behave erratically when dropped on water and why the
bristles of a paint brush cling together when it is lifted out of water. The motion of ripples on
a water surface is also governed by surface tension.
Surface tension is known to be
due to intermolecular attractions in the liquid surface and these forces produce a skin effect
on the surface. The forces between individual pairs of molecules are very small, and so in a
definition of the surface tension we consider the effect of a large number of molecules in a
line in the surface.
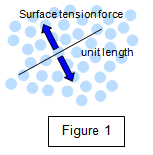
Consider a line of unit length drawn in the surface of the liquid
and think of the forces acting on the molecules in that line. Clearly the forces will act in all
directions in the surface but we will consider only those components of force acting at right
angles to the line (see Figure 1). The force on the whole line is the sum of all the forces on
the individual molecules. Notice that any given molecule is in equilibrium due to equal and
opposite forces acting on it.
The size of the force of surface tension in a liquid
surface is governed by a property called the coefficient of surface tension for that liquid.
The coefficient of surface tension of the liquid is defined as follows:
| Liquid | Surface tension (Nm-1x103) | Liquid | Surface tension (Nm-1x103) | |
| Methylated spirits | 22.6 | Ethyl alcohol | 22.3 | |
| Glycerol | 63.4 | Turpentine | 27 | |
| Water | 72.7 | Bromine | 41.5 | |
| Benzene | 28.9 | Acetone | 23.7 | |
| Mercury | 472 | |||
| Olive oil | 32 |
The
surface tension of a liquid decreases with increase in temperature and vanishes at the
critical temperature. All values given in the table are for temperatures of 20 oC, except that
for gold which is for 1130oC.
The following observations can be explained in terms
of surface tension:
(a) A pond-skater can walk on water but a person cannot.
(b) A
wet tent will let in water if the inside is touched.
(c) Water will rise up the capillaries
inside a plant stem (surface tension only partly explains this effect, however).
(d) A
needle may be made to float on water.
(e) A small piece of soap fixed to the back of a
piece of cardboard that is floating on water will cause the cardboard to move over the water
surface.
(f) Lead shot is made by pouring a molten stream of lead from a tall
tower.