
Rubber is a polymer and so consists of many long chain molecules. When the rubber is in an unstretched (relaxed) state these molecules are tangled up as shown in Figure 1(a). As a steadily increasing force is applied these molecules begin to straighten out (Figure 1(b)) – the bonds between adjacent chains are broken. These bonds are relatively weak compared with the bonds along each chain molecule. During this untangling and while the bonds are breaking the rubber warms.
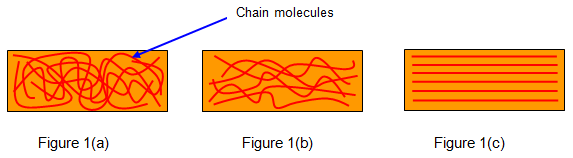
Eventually all the
molecules have been straightened (Figure.1(c). Up to this point it is
quite easy to extend the rubber because all that was being done was to
untangle the chains and break the weak bonds between them. However when
the molecules are straightened it becomes much more difficult. This state
can be noticed easily by using a rubber band since when this point is
reached the surface of the rubber becomes whiter and rougher.
As the rubber is
allowed to relax by slowly removing the force the chains of molecules
intertwine again but the cross links do not completely reform. The heat
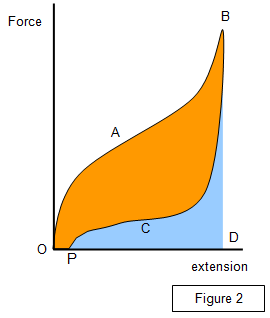
energy produced during stretching is not recovered. Plotting a graph of
force against extension the amount of energy converted to heat within the
specimen can be found. (Figure 2).
Area: OABDO - energy
given to band during stretching
Area: PCBD - energy released from band during
contraction
Area: OABCPO - energy converted to heat within the band
OP
- permanent extension of band
Using a rubber band about 2mm wide and
20cm long and a maximum load of 15N the energy converted to heat is of
the order of 0.5 - 1 J.