
As you know
there are two kinds of particle in the nucleus of an atom, protons, carrying a unit positive charge
and neutrons which are uncharged. It is therefore pretty surprising that the nucleus holds together
at all - you would expect the electrostatic repulsion from all those positively charged protons to
blow it apart. The fact that this doesn't happen is very good evidence for the existence of another
attractive force between the nucleons.
This is called the strong nuclear force. It only acts over very short distances (10-
15 to 10-14 m), and it is what holds the nucleus together.
In small nuclei the
strong force from all the nucleons reaches most of the others in the nucleus but as we go on
adding protons and neutrons the balance becomes much finer. The longer range electrostatic
force affects the whole nucleus but the short-range strong nuclear force of any particular nucleon
only affects those nucleons around it - the rest of the nucleus is unaffected. The nucleons are not
held together so tightly and this can make the nucleus unstable. (See nuclear fission).
However the more protons there are in a nucleus the greater the electrostatic forces
between them and we need a few extra neutrons to help "keep the protons apart". This is why
heavy nuclei have more neutrons than protons.
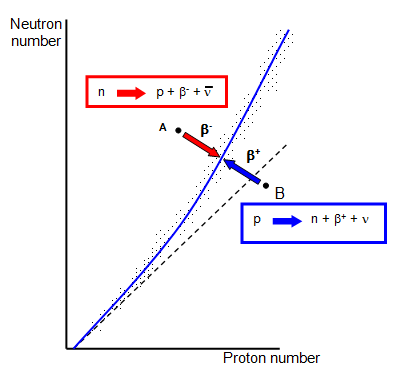
The variation of neutron number with proton number is shown in the graph. You can
see that for light elements these two numbers are the same but become very different for heavy
elements. Adding more neutrons helps to keep the nucleus stable but when the number of protons
is greater than 83 adding more neutrons is not enough and all elements with a proton number of
greater than 83 are unstable.
Nuclei that lie on or near the central line are stable but as
you move to either side of that region they become unstable.
They can return to the stable
state by emitting either a positron (side B) or an electron (side A).