Conductivity of electrolytes
AIM
The aim of this experiment is to measure the conductivity of an electrolyte. Using a dc source would lead to polarisation so an oscillator and headphones (or an oscilloscope) replace the cell and galvanometer.
YOU WILL NEED
A standard resistor of between 10Ω and 20Ω, a metre wire bridge, a jockey, a signal generator, leads, headphones or an oscilloscope, a conductivity cell, liquids
WHAT TO DO
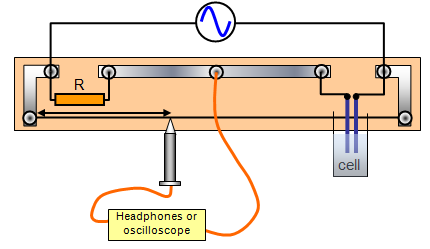
Set up the apparatus as shown with the first of your sample liquids in the conductivity cell.
Adjust the oscillator to about 800Hz. Move the jockey until the sound in the headphones is zero or a minimum.
Measure the dimensions of the cell (i.e. the area of plates A and the plate separation L).
ANALYSIS AND CONCLUSION
Use the standard equation for a Wheatstone Bridge to calculate the resistance of the conductivity cell (R).
Hence calculate the conductivity of the electrolyte from the equation:
Conductivity = L/[RA] Sm
-1
Repeat the experiment with the other available liquids.
An alternative version of this experiment would be to use either a resistance probe and data logger or a conductivity meter.

| SAFETY CONSIDERATIONS: There are no significant safety considerations in this experiment. |
Note: These instructions are only intended as an outline of experimental procedure. You should consult your teacher for a more detailed version before carrying out the experiment.