
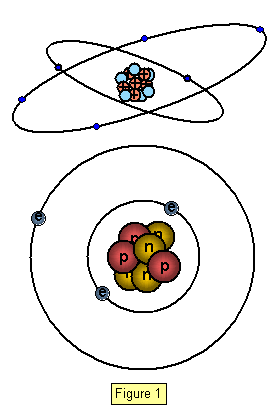
In an atom the electrons
orbit the nucleus with the ones in the orbits more distant from it having the greater energy.
We call these orbits energy levels and when an electron moves from one level to another its
energy changes.
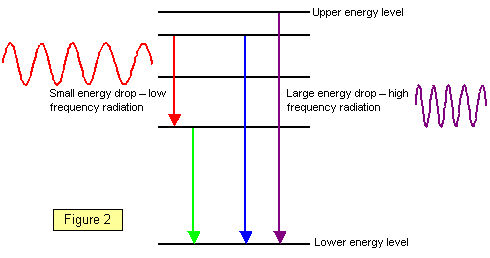
If an electron falls from a
high energy level to a low energy level energy is given out as electromagnetic
radiation. The greater the difference in energy between the two levels the greater the output
of energy.This energy is emitted as a
small 'bundle' of energy called a photon. The bigger the energy drop the higher the
frequency of the electromagnetic radiation. (See Figure 2).
The greater the number of photons emitted per second the brighter the light.