
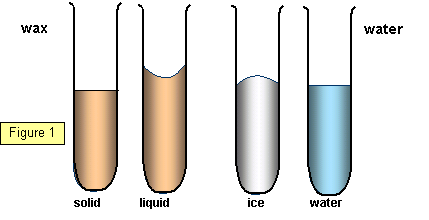
The volume of the solid is usually
smaller than the liquid from which it was formed, a good example of this is wax. However,
water is odd, its volume actually increases when it freezes.
(See Figure
1)
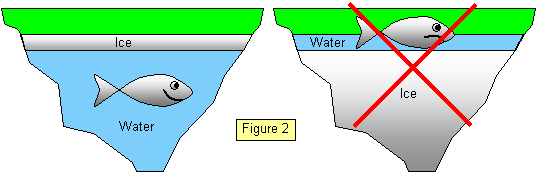
This strange behaviour is very important. As ice has a greater volume
than water it is less dense than the water and so it floats, this means that ponds freeze from
the top downwards. If this were not so the oceans would be solid ice to quite close to the
surface!
At 4oC water has its greatest density and so it will sink to the bottom of the
pond. The water above will be colder until just below the ice it is at 0oC. (The top of the ice
may be well below 0oC.) (See Figure 2)
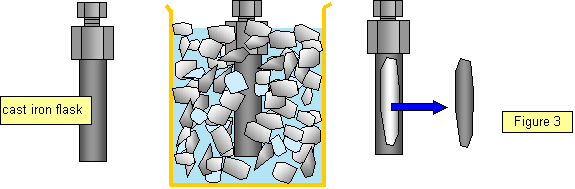
You can show this effect by filling a cast iron flask with water, fitting the screw top firmly in place and then putting it in a freezing mixture so that the water in the flask freezes, The increase in volume of the water as it turns to ice is enough to shatter the flask. (use a plastic beaker to hold the freezing mixture!) (See Figure 3)

If the water in a pipe freezes the volume of the ice will be greater than
the volume of the water and this will exert an enormous pressure of the pipes. This often
causes the pipes to burst – you only find out about a broken pipe after the ice has melted
again and the water runs out of the cracks! The photograph of central heating pump that burst when the water in
it froze (the air temperature had fallen to -7oC) shows how great these forces
are.
The split in the cast iron pump casing can clearly be seen at the top of the
pump.
If we add salt to an ice/water mixture it will cool well below 0oC, the
normal freezing point of water. This freezing mixture can reach as low as -
18oC.
Adding the salt to the ice lowers its melting point and as the ice is at 0oC,
which is above its new freezing point, it melts and absorbs latent heat from the mixture and
so the whole mixture cools.
Ice can be melted on pavements in this way. If salt is
added the temperature falls to the freezing point of the ice/salt mixture and this mixture will
stay a liquid.
Solder is an interesting example of this effect. Solder is a mixture of tin
and lead and its melting point is lower than that of either of the pure
metals.
The
melting point of ice may also be changed by a change in pressure. If we increase the
pressure on the ice its freezing point will be lowered. The change in pressure has to be large
to show any effect - doubling the atmospheric pressure on some ice will only lower its
freezing point by less than one hundredth of a degree.
An increase in pressure lowers
the melting point of ice.
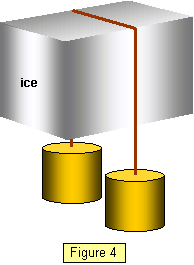
You can do a simple experiment to show this.
If
you hang two one kilogram masses from the end of a piece of 24 SWG copper wire over a
block of ice an interesting thing happens. The wire slowly cuts its way through the block and
the water refreezes above the wire. (Figure 4)
This is explained by the increased
pressure below the wire melting the ice as it lowers its freezing point.
This change
of melting point with pressure is called REGELATION.
The latent heat comes from
the copper wire and the heat produced on freezing is conducted through it. The experiment
works very slowly with iron wire and not at all with string. It will not work with string because
the string does not conduct the heat away and although the string will cut into the ice there
will be no refreezing above the string.
Glaciers move because the enormous
pressures underneath them melts the ice and so they slide along on a film of water. Ice
skating probably does not; the film of water under the skate is probably produced by the heat
of the friction between the skates and the ice as the skater moves over it.
Snowballs are
hard to make on very cold days because you can't produce enough pressure to melt the
snow and make them stick together.
Antifreeze in car radiators has just the same
effect. The more antifreeze, the colder the water has to be before it will
freeze.