

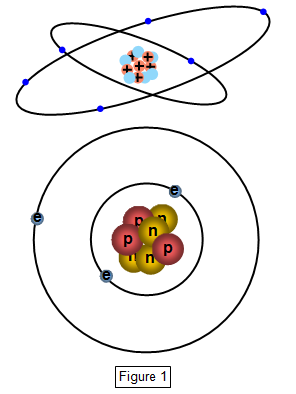
A simple view of the inside of an atom is shown in Figure 1. The electrons
orbit the nucleus with the ones in the orbits more distant from it having the greater energy.
We call these orbits energy levels and when an electron moves from one level to another its
energy changes.
To move an electron from an inner orbit (low energy level) to an
outer orbit (higher energy level) requires an input of energy while if an electron falls from a
high energy level to a low energy level energy is given out. This energy is electromagnetic
radiation. The greater the difference in energy between the two levels the greater the output
of energy.
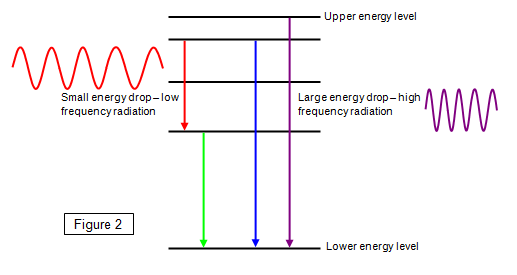
Every time an electron falls from one energy level to another it emits a
small 'bundle' of energy called a photon. The bigger the energy drop the higher the
frequency of the electromagnetic radiation. (See Figure 2).
The brightness of the
source of light depends on the number of these photons emitted per second. The greater the
number the brighter the light.
Each photon has a tiny energy and a 100 W lamp will
emit roughly 100 million million million photons every second! Although they travel at the
speed of light (300 000 km/s) they are so tiny that we don't feel them hitting us. However the
radiation pressure from the impact of many millions upon millions of photons does have an
effect on the tail of a comet – helping to keep it pointing away from the Sun.