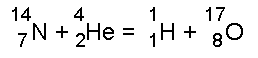


The idea of atoms as small particles was put forward by the Greeks 2000 years ago, but the structure of the inside of the atom was not understood until the beginning of the twentieth century!

The English scientist Thomson suggested that the atom, which is a neutral particle, was made of positive charge with 'lumps' of negative charge inset in it - rather like the plums in a pudding. For this reason it was known as the Plum Pudding theory of the atom. (Figure 1)
However, there were problems with this model but nobody could think of
a better one. Then in 1911 Rutherford, Geiger and Marsden were studying the passage of
alpha particles through thin pieces of gold foil. As they expected, some of the alpha particles
were able to pass through the film. They then noticed something that they did not
expect.
Some of the alpha particles were being deflected from their original path
and more surprising still about 1 in 8000 were actually knocked backwards! You must
remember that although alpha particles are very small they are travelling at a tenth the speed
of light.


Rutherford explained it this way. He knew
that the alpha particles carried a positive charge so he said that the positive charge of the
atom was concentrated in one place that he called the nucleus, and that the negatively
charged particles, the electrons, were in orbit around the nucleus.
The atom is
mostly empty space! Think of a small marble with a cloud of gnats round it in a sports hall.
That is like the nucleus, electrons and the volume of the whole atom.
Figure 3
shows the alpha particle scattering explanation that led to the modern idea of the structure of
the atom.