Mean free path of molecules in a gas
The molecules in a gas are
assumed to be in continuous random motion making collisions with each other and with the
walls of the container. The mean distance that the molecules travel between one collision
and the next is called the mean free path of the molecules.
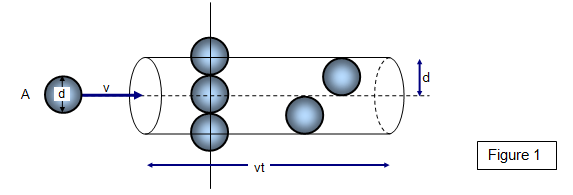
Consider a molecule A with an effective diameter
d travelling with a velocity v (see Figure 1). In a time t the molecule will travel a distance vt
and collide with π d
2vtn molecules, where n is the
number of molecules per unit volume (Figure 1). The molecule collides with all molecules
whose centres are within a cylinder of length vt and cross-sectional area πd
2The mean free path is the total distance
covered (vt) divided by the number of collisions. Therefore the mean free path us given by:
Mean free path (L) = vt/[πd2nvt] = 1/[πnd2]
This proof assumes
that all the other molecules remain at rest. If we consider the case of moving molecules the
formula must be modified and becomes:
Mean free path (L) = = 1/√2[πnd2]
For air at 0
oC and at a pressure of
1 atmosphere d = 2x10
-10 m and n = 3 x 10
25 m
-3. This gives
a mean free path for air molecules in these conditions of 2x10
-7 m or 200 nm,
about a thousand molecular diameters. Each molecule makes about five thousand million
collisions per second!

At an
altitude of 100 km the mean free path is 1 metre and at 300 km it is nearly 10 km.
At
a depth of 5 000 m in a mine the density of air has risen to 2.32 kg m
-3 and the
mean free path is then reduced to about 75 nm.
A further practical study of the mean
free path in a gas may be made with the bromine diffusion experiment. In this experiment
bromine vapour is allowed to diffuse through air at atmospheric pressure in a closed tube.
The average distance d that the bromine diffuses in a certain time t is found by measuring
the progress of the 'middle brown' colour of the bromine gas.
 DANGER - ONLY TO BE
PERFORMED IN A FUME CUPBOARD BY A QUALIFIED TEACHER
DANGER - ONLY TO BE
PERFORMED IN A FUME CUPBOARD BY A QUALIFIED TEACHERNow it can be shown statistically
that d is related to the mean free path (L) of the gas by the formula:
d =
L[N]
1/2where N is the number of collisions made while the bromine is
diffusing through the distance d.
However, the total distance travelled by an indi
vidual molecule of bromine is D, where D = NL, and therefore:
Mean free path (L) = d2/D
Using the kinetic theory formula the velocity of the
gas molecules may be found and hence the total distance D travelled in a certain time T. The
mean free path can then be found.
Example problems
Using the data in the text calculate the number of air molecules per cubic metre (n) at the following places in the atmosphere.
(a) a height of 100 km
(b) a height of 300 km
(c) a depth of 5000 m in a mine.
Use: n = 1/√2[πLd2]
(a) n = 5.627x1018/1 = 5.6x1018
(b) n = 5.627x1018/104 = 5.6x1014
(c) n = 5.627x1018/75x10-9 = 7.5x1025