
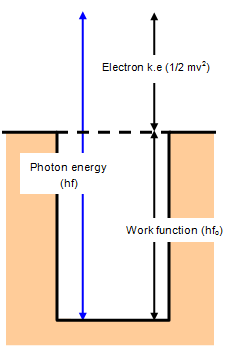
If a quantum of radiation with an energy greater than the work function
Eo, and therefore a frequency greater than fo, falls on a surface an electron will
be emitted with some kinetic energy and escape from the surface.
The kinetic energy of
the electron is then just the difference between the energy of the quantum and the work function of
the metal.
This can be expressed in the following equation - called Einstein's photoelectric
equation.
If we put a collecting electrode in front of the
emitting surface in a vacuum we can detect the photoelectrons as a small current. Changing the
frequency of the radiation will not have any effect on the current as long as it is bigger than
fo but increasing the intensity will increase the current.
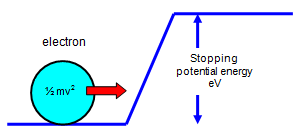
Now if we make the collecting
electrode slightly negative compared with the emitting surface the electrons will find it difficult to get
to it and electrons will only do that if their energy is greater than the "height" of the potential barrier.