Uses of radioactive isotopes
These materials have a variety of uses
and a selection of these are listed below.
(a) dating geological specimens, using
uranium, rubidium or bismuth;
(b) dating archaeological specimens, using carbon
14
(c) paper or plastic thickness measurement using beta radiation
(d) treatment of
tumours;
(e) sterilisation of foodstuffs;
(f) nuclear pacemakers for the
heart;
(g) liquid flow measurement;
(h) tracing sewage or silt in the sea or
rivers;
(i) checking blood circulation and blood volume;
(j) atomic lights using krypton
85;
(k) checking the silver content of coins;
(I) radiographs of castings and
teeth;
(m) testing for leaks in pipes;
(n) tracing phosphate fertilisers using
phosphorus 32
(o) smoke alarms
(p) sterilisation of insects for pest
control.
1. Radioactive dating
(a) Geological
dating
The dating of rocks is carried out using the decay of uranium 238 (half life
4.5x109 years), rubidium 87 (half life 4.7x109 years) or bismuth 40 (half life
1.3x109 years). The very long half-lives of these isotopes make them particularly
suitable for finding the age of rocks.
For example if you consider the uranium series that
the final stable isotope is lead-206, and if we assume that there was no lead in the rock when
it was formed the ratio of the number of atoms of lead 206 (NPb) to the number of atoms of
uranium 238 (NU) will give us the age of the sample.
Example problems
Consider a rock sample in which the ratio of lead-206 to uranium-238 is 0.7.
Now the initial number of atoms (No) is the sum of the numbers of lead(NPb) and uranium (NU) atoms present when the sample was analysed.
Therefore
NU = (NU + NPb)e-lt
where l is the decay constant for uranium-238 and t is the time that has elapsed since the rock was formed.
Therefore we can show that
NU = (NU + NPb)e-lt and so 1 = (1 + NPb/ NU)e-lt
1 = (1 + 0.7)e-lt
1.7 = e-lt and so lt = 0.53
But l = 5 x 10-18 s-1 and therefore t = 0.53/5x10-18 = 1.06x1017 s = 3.36x109 years
(b) Archaeological dating using carbon 14
The half-life of carbon-14 (5570 years) is
just the right sort of length for use in dating archaeological specimens with ages up to a few
thousand years.
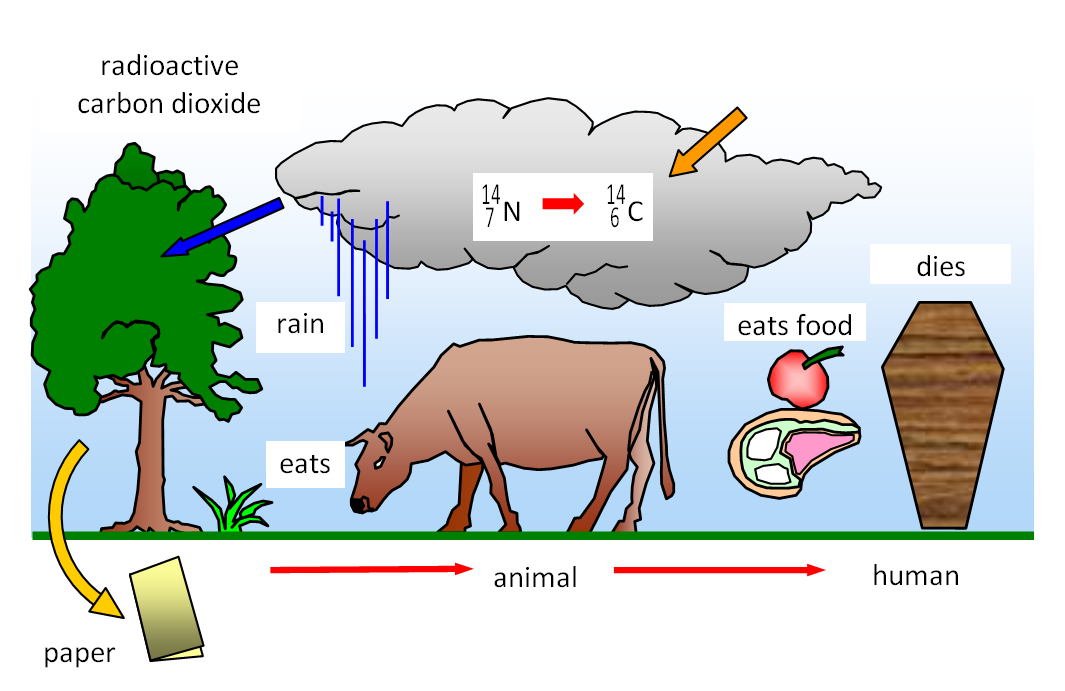
Carbon-14 is
continually being formed in the upper atmosphere. Cosmic rays can produce neutrons, and
the following reaction may then occur:
| 147N |
+ |
10n |
giving |
146C |
+ |
11H |
The carbon 14 is then absorbed by plants; these in turn
are eaten by animals which may then be eaten by other animals. As soon as the animal dies
the intake of radioactive carbon-14 stops and the proportion in the body starts to decrease.
Therefore if the proportion of carbon 14 to carbon 12 is known at the start, the age of the
specimen can be found once the amount of carbon 14 remaining in it has been measured. It
has been found that the activity of carbon 14 in living materials is about 19 counts per
minute per gram of specimen.
This method of dating can be used with success to
determine not only the ages of animal remains but also those of wood, paper, cloth and
other organic material.
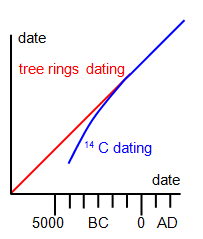
One difficulty with
this method is that it has to be assumed that the cosmic ray intensity has remained constant,
and in fact this has been found not to be the case. By comparison with the tree rings in the
extremely old bristle-cone pines, however, a corrected carbon date can be found for objects
over about 1500 years old. The trees are themselves dated by the carbon-14 method using
dead parts in the bark. A comparison between the carbon date and that due the tree rings is
shown in the diagram.
Example problems
A piece of bone from an archaeological site is found to give a count rate of 15 counts per minute. A similar sample of fresh bone gives a count rate of 19 counts per minute.
Calculate the age of the specimen.
The activity A of a sample is proportional to the number of radioactive atoms within it.
Therefore
A = Aoe-lt
giving 15 = 19e-lt
Therefore:
0.236 = lt
t = 0.236/l = [0.236 x 5570]/0.693
t = 1897 years.
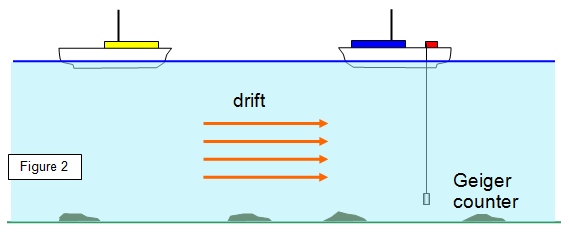
2. Radioactive tracers
If a little radioactive material is put into a moving liquid the path of this
liquid can be tracked. Used in testing blood flow, tracking underground streams and following the
movement of silt in rivers
4. Thickness gauge
A beta source is put on
one side of a sheet of material and a Geiger counter on the other. The amount of beta radiation
that gets through the sheet will give you an idea of its thickness.
5. Cracks in
castings
A gamma source is placed in a metal casting and a Geiger counter moved over its
surface. If there are any cracks in the metal gamma radiation can get through and be
detected.
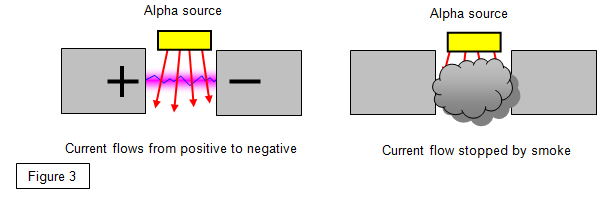
6. Smoke alarms
Many houses have a smoke alarm using a weak alpha
source. When smoke gets into the detector the alpha particles cannot get through to the sensor
and the alarm goes off.
6. Sterilisation of food
Bacteria in food can be killed if exposed to gamma radiation.
7.
Medical uses of radioisotopes
These fall into two main sections:
(i) diagnostic – where the
radiation is used to locate a problem
(ii) therapeutic – where the radiation is used to treat a
problem
(a) Cobalt-60 gamma ray sources of up to 10 000 curies have been used; such a source
gives 200 R per minute at 1 m. Treatment is typically 3 grays (<2 m) a day for 20 days. (1
gray = 100 rads.)
(b) Syringes and other medical equipment can be sterilised
using gamma radiation
(c) Certain radioisotopes called tracers will concentrate in specific
organs for analysis – for example iodine 131 in the thyroid and technetium 99 in the brain, lungs,
and red blood cells and can then be used to diagnose defects
(d) Iodine 131 is also used to
treat thyroid cancer (it has a half life of just over 8 days) and activity of 8 mC may be taken as
liquid or in a capsule.
(e) Iodine 123 (half life 13 hours) is
also injected into a patient where it concentrates in some organs and the gamma radiation emitted
by the source is scanned with a camera. It is especially suitable for medical studies since it gives no beta-
radiation.
(f) Technetium-99 with a half-life of 6 hours gives gamma-rays of
140 keV energy.
A VERSION IN WORD IS AVAILABLE ON THE SCHOOLPHYSICS USB