
Over the years scientists have tried to discover
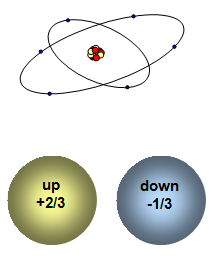
more and more about the atom. First the nucleus was discovered with its surrounding cloud
of electrons and then, as they probed deeper, the proton and neutron inside the
nucleus.
In the 1960's physicists began to wonder whether the proton and neutron
were really 'fundamental' particles or whether there were even smaller particles inside them.
In 1964 Murray Gell-Mann and George Zweig proposed the name quark for these particles. (The name rhymes with park).
It was found that there were two basic
types of quark – and these were called the up quark and
the down quark. Up quarks have a charge of +2/3 of the
size of the charge on an electron and down quarks have a charge of –1/3 the size of the
charge on an electron. The up and down quark were finally discovered in
1975.
(There are other quarks and if you want to find out about these see: Quarks in the 16-19 section of the
site.)
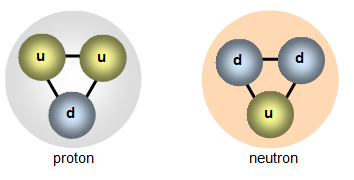
Both the neutron and proton are each made up of three quarks. The
proton has two up quarks and one down quark to give a charge of (+2/3) + (+2/3) + (-1/3) =
+1 and the neutron is made of two down quarks and one up quark to give a charge of (-1/3) +
(-1/3) + (+2/3) = 0.
The quarks are held together inside the
proton and neutron by a force called the strong nuclear force. This is a very large force but it
only acts over very small distances about the size of the nucleus. At the moment it has not
been possible to detect quarks outside the nucleus. The more you 'pull' to try and get a quark
out of a proton or neutron the bigger the force becomes pulling it back in again.
At
the present time (2010) quarks seem to be really 'fundamental' (basic) particles and not
made of anything others.
Remember that it is not really that there are three quarks
inside a proton like three marbles in a tin – the three quarks ARE the
proton!
We think that electrons and neutrinos are really fundamental particles
themselves. They do not contain any quarks.