
Rubber has some strange elastic properties.
It
is a polymer – that means that the rubber molecules are made of long chains of atoms.
When a rubber band is unstretched (relaxed state) the molecule chains are all twisted up
(Figure 1(a)). As the rubber is stretched they begin to untangle themselves as shown in
Figure 1(b).
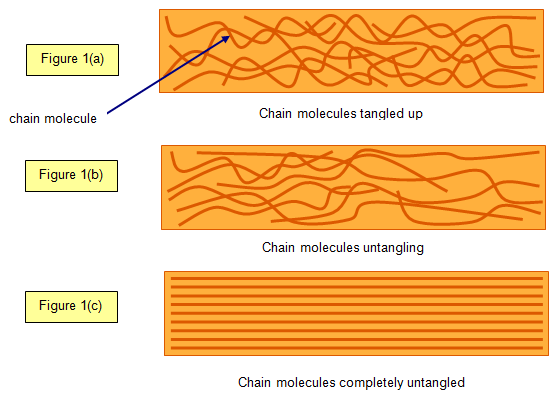
Finally they are
all lined up (Figure 1(c)). At this point the rubber changes its appearance – the surface looks
rougher and whiter. At the start it was quite easy to stretch the rubber band but now it
becomes much more difficult to stretch. This is because up to this point you have simply
been untangling the long chain molecules but when the molecules are straight they cannot
be untangled any more and what you are now doing is trying to actually stretch the
molecules themselves!
You may notice that when a rubber band is stretched it gets
slightly warmer. You can test this by taking a rubber band, holding a short length of it,
stretching it quickly and then holding it against your lips – it feels warmer. You may notice that when a rubber band is stretched it gets slightly warmer. You can test this by taking a rubber band, holding a short length of it, stretching it quickly and then holding it against your lips – it feels warmer. When the rubber band is stretched its molecules get straightened. This means a decrease in volume. This causes its temperature to increase just like an ideal gas, which heats up when it is compressed. If you then let it cool down while stretched and release it, it will get colder than room temperature. This is because its volume has increased, so the temperature drops - just like an ideal gas.
As the rubber is stretched the
bonds between adjacent chains are broken. These bonds are relatively weak compared with
the bonds along each chain molecule.
If you stretch a rubber band and record the
extension against load and carefully take the load off again still recording the extension you
can plot a graph like that shown in Figure 2.
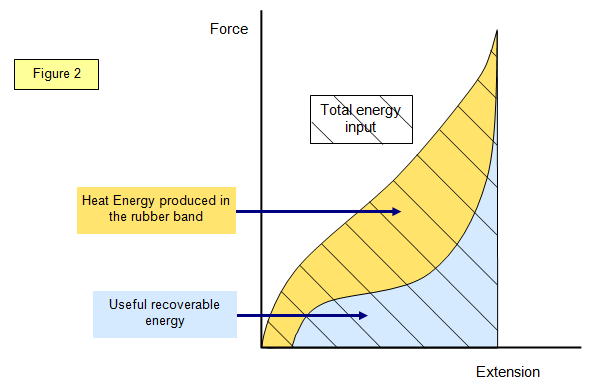
To work out the energy of each section you have to find
the area under the line. This is best done by drawing the graph on a piece of graph paper
and then counting the squares.
You can observe another very odd effect. Stretching
a rubber band makes it get hot – heat energy is lost. Therefore if you put heat energy into a
rubber band it will get shorter – unlike most materials when they are heated.
If you
play squash you may have wondered why the ball becomes much more bouncy after hitting
it around for a while. This is because it heats up, the air inside the ball also heats up, its
pressure increases and it becomes harder (like a well blown up tyre) and so will bounce
better.