

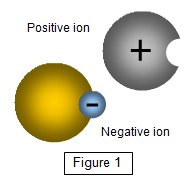
Ions are made when
atoms or molecules (linked groups of atoms) are literally torn apart to give positive and
negative ions or positive ions and free electrons. This may sound a bit violent but you can do
it by simply putting a voltage across the gas or liquid. This is what happens in electrolysis
where a voltage is applied to two metal plates in a liquid.
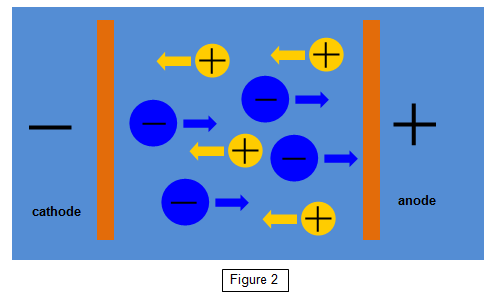
In the electrolysis of
copper sulphate the liquid is ionised to give positive copper ions and negative sulphate ions.
(Cu++ and SO4--). Two electrons are torn from the copper
atoms and added to the sulphate ions.
The positive copper ions are then attracted to
the negative electrode while the negative sulphate ions are pulled towards the positive
electrode (See Figure 2).
Ionisation of water will give you positive hydrogen
ions (H+) at the cathode and negative ions (OH-) at the anode.
Applying a high voltage
across a gas such as air will ionise the air molecules allowing a current to flow through it and
giving a spark. The spark is the movement of the positive and negative ions through the air.
You will have seen the ionisation of air on a large scale as lightning.
(See: Lightning)
Ionisation can also be
produced by charged particles such as alpha or beta radiation colliding with an atom or by
the impact of gamma radiation on the atom. The ions formed in one collision can move on to
collide with other atoms – ionising them to give what is called an avalanche of ions.