Expansion of gases 2
(a) any kind of pressurised gas canister will explode if heated over a fire and so can be very dangerous
(b) the gas/air mixture in the cylinder internal combustion engine "explodes" and expands when heated by a spark from the spark plug
(c) the air in a hot air balloon expands when it is heated so lowering its density and helping the balloon to rise
(d) an inflated lilo or rubber dinghy may explode if left in the sun for a long time
(e) a dent can be removed from a table tennis ball by putting it in hot water
(f) cakes "rise" because of the gas in them expanding
(g) Galileo's air thermometer works by the expansion of air
(h) racing car tyres are kept warm to make them expand and become firm. This raises the cars slightly off the road
(i) when a bullet is fired the air behind it is heated rapidly by the explosion of the cartridge and the bullet is forced up the barrel of the gun
All these effects can be explained by the expansion of gases.
When a gas is heated the molecules in the gas move round faster.
The more it is heated the faster they move and the hotter the gas
becomes. The molecules themselves do not get any bigger but because
they move faster they hit the walls of the container that they are
in at a greater speed and so try to expand the container. If it
can, the volume of the container increases. There is also another
effect – because the molecules are hitting the walls of the container
faster they cause a greater force on the container and so the pressure
of the gas in the container rises.
The expansion of a gas when it is heated can easily be shown by
the following simple experiments:
(a) heating a tin with a lid over a Bunsen flame (careful – safety
screen needed
(ONLY TO BE DONE BY A TEACHER)

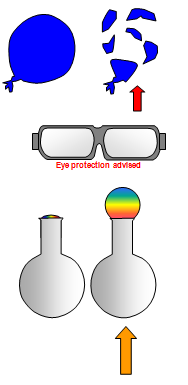
(b) heating a partly blown up balloon over a Bunsen (careful – it
will explode!)
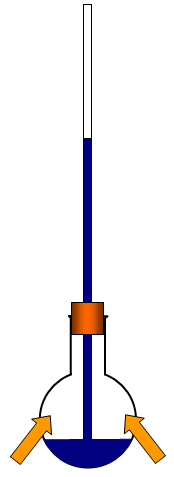
(c) make a soap film over the neck of a flask and then warm the
flask with your hands – you will make the film rise into a bubble
(d) pop corn gets larger when it is cooked because of the expansion
of the gas within it
(e) partly fill a flask with liquid and put a capillary tube in
it so that some of the liquid is forced up the tube. Warm the air
above the liquid in the flask with your hands or with a Bunsen –
the liquid will rise up the tube as the air expands.
SAFETY GOGGLES SHOULD BE WORN FOR (a) AND
(b)
A VERSION IN WORD IS AVAILABLE ON THE SCHOOLPHYSICS USB