
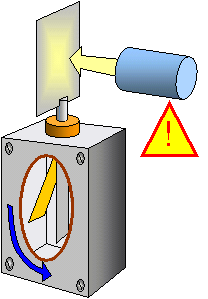
If a zinc plate connected to a gold leaf electroscope is negatively charged and illuminated with ultra violet light the leaf falls immediately, the electroscope has been discharged.
(a) no electrons are emitted from the plate if it was positive
(b) the number of electrons emitted per second depends on the intensity of the incident radiation
(c) the energy of the electrons depends on the frequency of the incident radiation
(d) there is a minimum frequency (fo) below which no electrons are emitted no matter how long radiation falls on the surface
The quantum theory of Max Planck explains the photoelectric effect. It states that all radiation is emitted in quanta and the energy of one quantum is given by the equation:
The energy needed to just release a photoelectron is known as the work function of the metal.