Isotopes
|
Isotopes are particles which have the same position in the Periodic Table, that is, they are atoms of the same chemical element but with different nucleon numbers. |
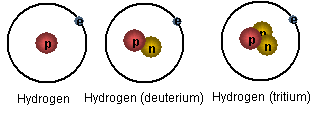
Isotopes of an element have nuclei with the same number of protons but different numbers of neutrons.
Neon, for instance, has three isotopes with nucleon numbers of 20, 21 and 22, corresponding respectively to 10, 11 and 12 neutrons in the nucleus.
Since the number of electrons is identical for all isotopes of the same element, the chemical properties of isotopes of the same element are identical. However since their relative atomic masses are different, some of their physical properties will also be different.
For example, the boiling point of ‘heavy water’ (water containing the isotope of hydrogen with a neutron in the nucleus) is 104
oC.
| Element |
Isotope nucleon numbers |
| Hydrogen |
1, 2, 3 |
| Helium |
3, 4, 5 |
| Carbon |
12, 14 |
| Oxygen |
16, 17, 18 |
| Neon |
20, 21, 22 |
| Uranium |
235, 238 |