
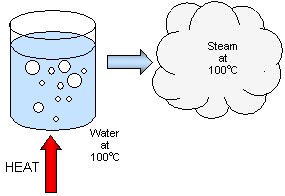
The energy needed to change the state of a substance from a solid to liquid or from a liquid to a gas heat energy is called LATENT HEAT energy.
This energy is used not to heat up the substance but to separate the molecules from each other.
While a solid is melting and while a liquid is boiling there is no temperature change.
The amount of heat needed to change the state of 1 kg of a substance is called the SPECIFIC LATENT HEAT of the substance.
The units for specific latent heat are joules/kilogram (J/kg).