
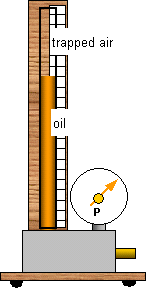
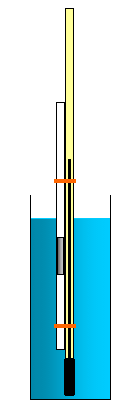
If we combine Boyle's Law and Charles' Law we get another equation involving pressure, volume and temperature.
This equation is called the gas equation or more properly the Ideal Gas Equation.
The quantity R is the universal gas constant and its value is 8.3 J/mol K.
Remember that in all these gas calculations the temperature must be
given in Kelvin (oC + 273)