
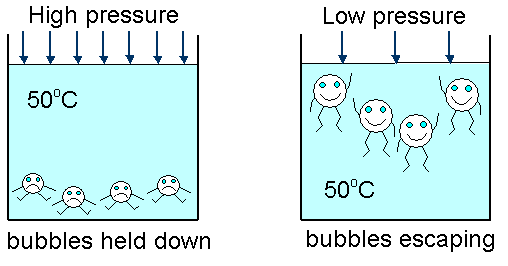
When a liquid boils, bubbles of gas rise from within the body of the liquid and escape from the surface. This effect occurs at one precise temperature for a liquid.
Adding impurities to a liquid raises its boiling point - adding salt to water.
Increased pressure also raises the boiling point.
Decreased pressure lowers the boiling point.