
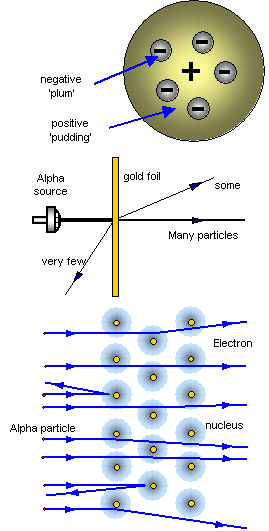
The idea of atoms as small particles was put forward by the Greeks 2000 years ago. In the 1890s J.J.Thomson suggested that the atom was made of positive charge with ‘lumps’ of negative charge in it - like the plums in a pudding.
It was known as the Plum Pudding theory of the atom.
Then 1911 Rutherford, Geiger and Marsden were studying the passage of alpha particles through thin pieces of gold foil.
Some of the alpha particles were able to pass through the film, but some were being deflected from their original path and more surprising still about 1 in 8000 were actually knocked backwards!
Rutherford knew that the alpha particles carried a positive charge so he said that the positive charge of the atom was concentrated in one place that he called the nucleus, and that the negatively charged particles, the electrons, were in orbit around the nucleus.
The atom is mostly empty space! The nucleus and electrons take up only a small part of the volume of the whole atom.
An atom consists of a heavy positive central nucleus surrounded by a cloud of negative electrons.