
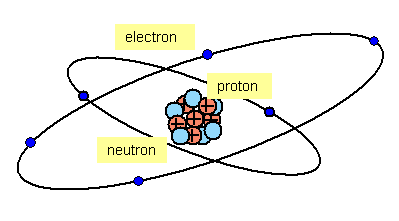
All atoms consist of two parts:
(a) a central heavy NUCLEUS that contains:
(i) PROTONS – particles with a unit positive charge
(ii) NEUTRONS - neutral particles with a mass slightly greater than that of a proton
(b) ELECTRONS - negatively charged particles orbiting the nucleus
ATOMS ARE NEUTRAL PARTICLES - THEY HAVE NO NET CHARGE
The total number of NEUTRONS and PROTONS in a nucleus is called the NUCLEON NUMBER
The number of PROTONS in the nucleus is called the PROTON NUMBER
The number of NEUTRONS in the nucleus is called the NEUTRON NUMBER