
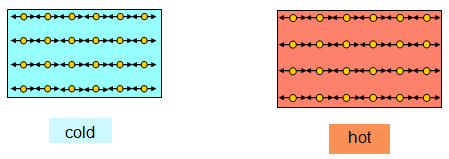
In a solid each molecule vibrates about a mean position. The hotter the solid the more violently the molecules vibrate.
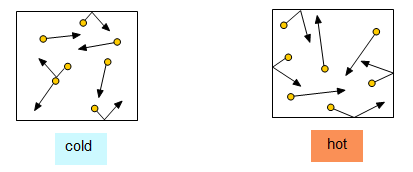
In a gas the molecules are all moving about randomly and at high speed. At room temperature they travel at around 400 m/s. The molecules must be moving faster than the speed of sound because it is just these moving molecules that transmit sound. Heating the gas makes the molecules move around more violently while cooling the gas makes the molecules slow down.
If you
keep cooling the gas the molecules move slower and slower and have less and less
kinetic energy. In the end they would stop – zero kinetic energy and so zero
temperature. This temperature is called ABSOLUTE
ZERO and it value is –273oC. We do not believe that it is
possible to reach absolute zero although scientists have got close to this, down to
0.000 001 oC above that temperature.
As the molecules move
around they collide with both each other and any solid object. If the gas is in a
container the molecules collide with the walls of the container at high speed. It is these
collisions that result in the pressure of the gas
If you compress the
gas the molecules hit the walls of the container more often – this means an
increase in pressure.
If you heat the gas the molecules collide
with the walls more violently – this also increases the gas pressure.
The
reverse is also true – expansion and cooling result in a decrease in
gas pressure.
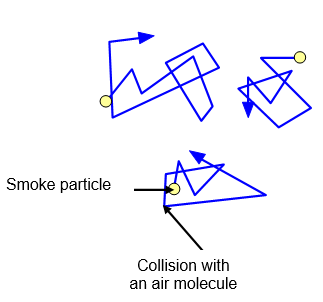
One of the best ways of showing that air
molecules are moving is known as Brownian motion. Some smoke is blown into a
small glass pot containing air and then viewed through a microscope. The air
molecules collide with much larger smoke particles and if a strong light is shone on the
box the smoke particles can be seen. They judder around as they are hit from all sides
by the much smaller and invisible air molecules. The particles can be seen because
light is scattered from them and they look like tiny stars.
(Compare with the
polystyrene ball in the kinetic theory model apparatus using small ball bearings to
represent air molecules).
If some bromine is released into a tube of air the
colour of the bromine gas can be seen spreading slowly through the air – this is
diffusion. The air molecules affect the movement of the bromine molecules. If the
experiment is repeated in an evacuated tube the spread of colour is virtually
instantaneous.