
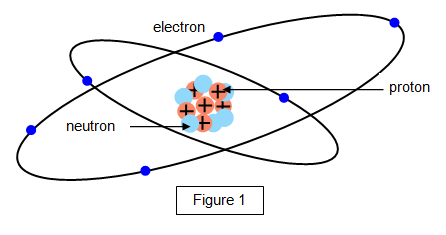
As you know atoms are very small but every atom has even
smaller particles inside it.
All atoms consist of two parts:
(a) a central heavy NUCLEUS
that contains
(i) PROTONS – particles with a unit positive charge
(ii) NEUTRONS -
neutral particles with a mass slightly greater than that of a proton
(b) ELECTRONS orbiting the
nucleus. These are particles with a negative charge, equal and opposite to that of a proton. They
have a mass about 1/1860 of that of a proton.
We really ought to explain what we mean by
light and heavy here.
You would need 1000 million million million million protons or neutrons to
have a mass of one kilogram. This may seem a huge number but you would need almost 2000
times as many electrons to have the same mass!
The diagram below shows a simplified
picture of the structure of an atom.

Atoms are also very small. We can think of an atom as a tiny sphere about a
hundredth of a millionth of a centimetre in diameter.
This means that 10 thousand million
atoms could be laid side by side along a one metre rule!
The number of PROTONS in the
nucleus tells us what element the atom is:
If there are six it is carbon
If there are eight it is
oxygen
If there are twenty-six it is iron
If there are ninety-two it is uranium
For
normal atoms the number of protons is balanced by an equal number of orbiting electrons - this
makes the atom as a whole neutral.
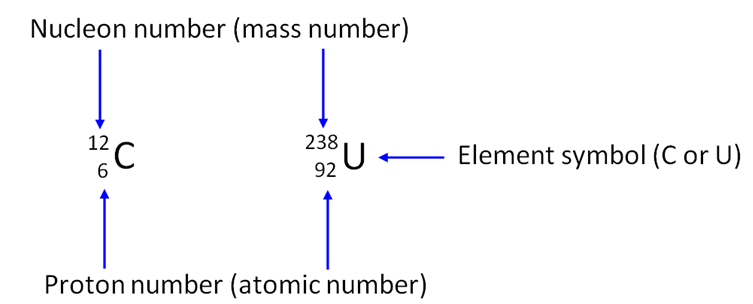
This means that in the
nucleus of a carbon atom there are 6 protons and 6 neutrons making 12 particles (or nucleons). In
a uranium atom there are 92 protons and 146 neutrons making 238 nuclear particles
(nucleons).
You will find a list of the structure of some of the more common elements in
the data section.
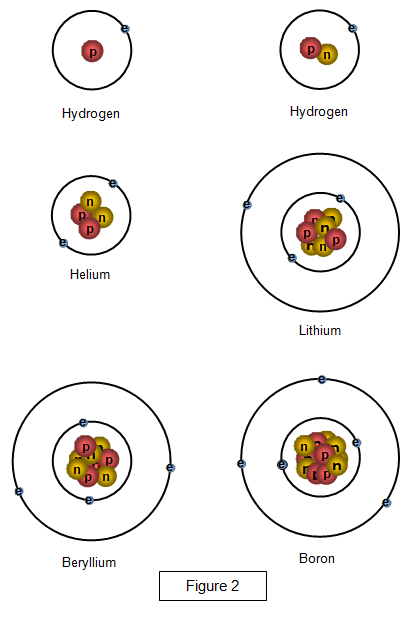
The diagrams below show the structure of a few of the lighter atoms.