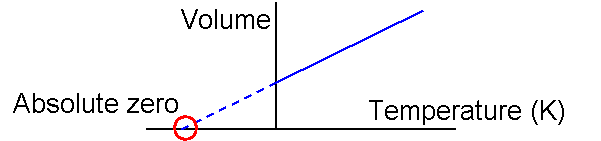

When a gas is heated the molecules in the gas move round faster.
The molecules bang into each other and into the walls of the container more violently and this increases the pressure. The gas will expand if it can.
Volume of the gas/Absolute temperature of the gas = constant (at a constant pressure)